Ariana Symbiont Integration Project Physiological Samples Collected in July 2020
Processing Ariana’s Montipora capitata Larval Development Physiological Samples from July 2020
Goal:
Process all of the physiological data (i.e. counts, size, symbiont counts) for the Montipora capitata (vertical spawner) developmental timepoint samples (n=40). Samples were collected at the Hawaii Institute of Marine Biology in July 2020 and are apart of a larger project which focuses on early life history energetics and symbiotic integration in vertical and horizontal transmission corals.
Contents
Count Protocol
Materials
- Glass pipette
- OMAX compound light microscope
- 70% ethanol solution
- 3 cell culture dishes
- Hand held tally counter
Protocol Steps
- I removed the physiological larval samples (H1-40) from the -4 °C fridge behind the titrator.
- I carefully used 70% ethanol and the glass pipette to remove all of the contents of each tube into a cell culture dish.
- I first counted one of the fertilized egg replicates by hand to get a total of 1,047 fertilized eggs total in sample H1. After discussing with Ariana, we decided to take a subset (roughly 200-300 fertilized eggs) of samples that had large amounts in them. The first 8 samples (H1 to H8) had large amounts so I labeled new tubes (ex: H1_1, H2_1, H3_1….H8_1) and took a subset sample (~200-300).
- To count each sample, I repeated step 2 and then counted all the individuals in each tube moving the contents in one cell culture dish to the next whilst keeping track of counts using the hand held tally counter.
- If I was ever concerned that individuals were stuck together or too small to count by eye, I placed the cell culture dish under the 4x magnification on the compound light microscope.
- In between each count, I cleaned the glass pipette and cell culture dishes with the 70% ethanol solution.
Count data for all samples (n=40) completed on 20210304
| tube.ID | lifestage | date | num.sampled | count | initials | notes |
|---|---|---|---|---|---|---|
| H1-1 | Egg Fertilized | 20210304 | subset | 356 | DMB | |
| H2-1 | Egg Fertilized | 20210304 | subset | 259 | DMB | |
| H3-1 | Egg Fertilized | 20210304 | subset | 256 | DMB | |
| H4-1 | Egg Fertilized | 20210304 | subset | 269 | DMB | |
| H5-1 | Embryo 1 | 20210304 | subset | 254 | DMB | |
| H6-1 | Embryo 1 | 20210304 | subset | 232 | DMB | |
| H7-1 | Embryo 1 | 20210304 | subset | 306 | DMB | |
| H8-1 | Embryo 1 | 20210304 | subset | 214 | DMB | |
| H9 | Larvae 1 | 20210304 | full.sample | 218 | DMB | some slightly degraded |
| H10 | Larvae 1 | 20210304 | full.sample | 294 | DMB | some slightly degraded |
| H11 | Larvae 1 | 20210304 | full.sample | 189 | DMB | some slightly degraded |
| H12 | Larvae 1 | 20210304 | full.sample | 119 | DMB | some slightly degraded |
| H13 | Larvae 2 | 20210304 | full.sample | 105 | DMB | |
| H14 | Larvae 2 | 20210304 | full.sample | 38 | DMB | |
| H15 | Larvae 2 | 20210304 | full.sample | 49 | DMB | |
| H16 | Larvae 2 | 20210304 | full.sample | 40 | DMB | |
| H17 | Larvae 3 | 20210304 | full.sample | 66 | DMB | |
| H18 | Larvae 3 | 20210304 | full.sample | 88 | DMB | |
| H19 | Larvae 3 | 20210304 | full.sample | 76 | DMB | |
| H20 | Larvae 3 | 20210304 | full.sample | 38 | DMB | |
| H21 | Larvae 4 | 20210304 | full.sample | 208 | DMB | |
| H22 | Larvae 4 | 20210304 | full.sample | 118 | DMB | |
| H23 | Larvae 4 | 20210304 | full.sample | 107 | DMB | |
| H24 | Larvae 4 | 20210304 | full.sample | 176 | DMB | |
| H25 | Larvae 5 | 20210304 | full.sample | 55 | DMB | |
| H26 | Larvae 5 | 20210304 | full.sample | 48 | DMB | |
| H27 | Larvae 5 | 20210304 | full.sample | 40 | DMB | |
| H28 | Larvae 5 | 20210304 | full.sample | 47 | DMB | |
| H29 | Larvae 6 | 20210304 | full.sample | 16 | DMB | |
| H30 | Larvae 6 | 20210304 | full.sample | 19 | DMB | |
| H31 | Larvae 6 | 20210304 | full.sample | 21 | DMB | |
| H32 | Larvae 6 | 20210304 | full.sample | 16 | DMB | |
| H33 | Recruit 1 | 20210304 | full.sample | 13 | DMB | |
| H34 | Recruit 1 | 20210304 | full.sample | 19 | DMB | |
| H35 | Recruit 1 | 20210304 | full.sample | 20 | DMB | |
| H36 | Recruit 1 | 20210304 | full.sample | 33 | DMB | |
| H37 | Recruit 2 | 20210304 | full.sample | 10 | DMB | |
| H38 | Recruit 2 | 20210304 | full.sample | 12 | DMB | |
| H39 | Recruit 2 | 20210304 | full.sample | 6 | DMB | |
| H40 | Recruit 2 | 20210304 | full.sample | 7 | DMB |
Notes for count data completed on 20210304
- Noticed that the larvae 1 timepoint replicates were slightly degraded but still had a sufficient amount of larvae that was good
Size Protocol
Materials
- Glass pipette
- Dissecting light microscope
- OMAX digital microscope camera
- ImageJ software
- OMAX 0.01 mm stage micrometer
- ToupView software (windows operating system only)
- Cell culture dish
Protocol Steps
- I used a glass pipette to carefully remove ~20-30 individuals from each of the sample tubes.
- I aligned the cell culture dish underneath the dissecting microscope with the light on medium to high and positioned the cell culture dish next to the 0.01 mm stage micrometer so that the total length of the scale could be seen within the photo along with all the individuals.
- make sure to keep some ethanol/water in the sample to reduce reflections of light and to have a more even photo
- it is very important to have the scale micrometer within each individual photo as the zoom and focus may change on the microscope in between samples and you will need it to calibrate your photos later on in ImageJ

- Once the stage micrometer and individuals were focused and clear in view, I used the ToupView software to snap a photo and save it to the windows computer.
- I uploaded the photos to Google Drive and then when I was ready to size the individuals I would open them in ImageJ.
- Refer to the ImageJ manual with any questions on how to calibrate or how to use the freehand tool
- I opened (File > Open) each individual photo into ImageJ and used the text tool to randomly select and label a sub-sample of 10 individuals per photo.
- make a small text box next to each individual you will size and label from 1-10, press command D after each text box to overlay it on the photo
- save the labeled photo before calibrating the scale in ImageJ
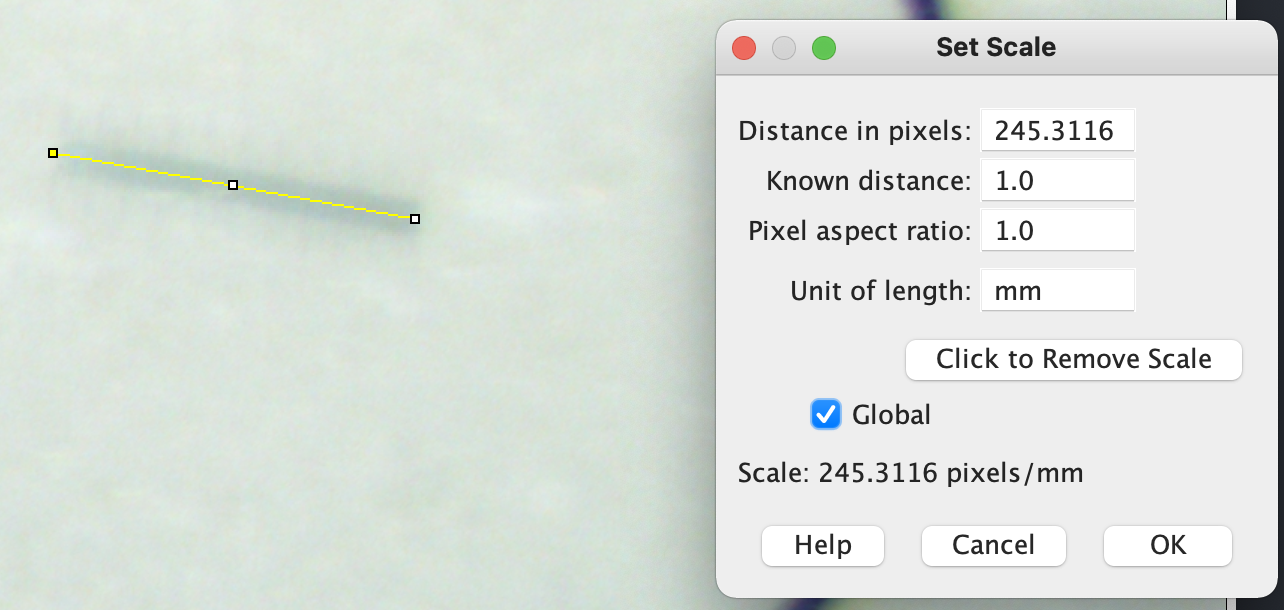
- I selected the Straight Line Selection Tool in ImageJ and zoomed in to the scale bar on the stage micrometer. I then carefully drew a line from each end of the 0.01 mm scale bar. I then set the number of pixels to a known distance of 1.0 and the unit length to mm. I also click on global so that it will be set for as long as I have this image open.
- I then use the freehand selections tool to carefully draw a circle around the entire outline of each individual.
- make sure to go slow and zoom in as far as possible with the individual still in focus when doing this step.
- After outlining the individual, I press command M (measure) and a small data box will pop up with the calculated surface area of the individual in mm.
- make sure to keep an eye on these measurements and if you see them fluctuating or having large numbers, do the set scale step above again
- I then save the photo again and transfer the measured values into a csv document.
- All labeled images were uploaded here.
Symbiont Count Protocol
I had to first troubleshoot various methods so that we could properly homogenize the eggs to larvae developmental stage samples. I had to ensure that the symbiont cells were not lysing or degrading due to the sonicator. I used 1 mL aliquots of Emma’s adult Mcap (n = 2) and Pacuta (n = 1) tissue homogenate samples and Hollie’s Mcap developmental timepoint samples (n = 6) to test sonicator and homogenizing methods.
Materials
- Glass pipette
- OMAX compound light microscope
- Vortex
- Haemocytometer
- Hand held tally counter
- Sonicator
- Transformer
- 1000 uL pipette
- Combination tube rack
Protocol Steps
Followed the E5 Symbiodiniaceae Cell Density Counting Protocol with a few modifications for sonicator testing.
- Since the sonicator had a 220V outlet intended for use in Mo’orea, we had to order a transformer to convert the voltage safely.
Always read the instructions carefully when operating a transformer and make sure there is no liquid around the transformer
- Once unpacked, I plugged the transformer into a 110V outlet and read the instructions carefully. I changed the INPUT to the transformer to 110V (this step is extremely important, make sure the voltage you plug the transfromer into matches the INPUT tab located in the back of the transformer).

- With the transformer turned to OFF, I plugged in the sonicator into the 220V outlet on the transformer and tested that it turned on with no noise or spark.

- Following the sonicator instruction manual, I filled the sonicator 2/3rds of the way which was 2,000 mL.
- This specific sonicator does not have a way to change the frequency settings, you can only adjust the time it runs for and the temperature it runs at.

- Placed a combination tube rack with the 1.5 mL tube size facing up to hold the test tubes while the sonicator runs.

- Tested the sonicator by adjusting the time setting in increasing intervals and using subsequent counts to make sure cells were not being lysed or degraded.
Symbiont count data for test samples completed on 20210321
- I used Emma’s adult tissue homogenate Pacuta and Mcap samples for this first round.
| tube.ID | time.sonicated | date.counted | num.squares | count1 | count2 | count3 | count4 | count5 | count6 | count7 | count8 | # individuals | volume (uL) | avg.per.square | cells.uL | avg.per.individual | std.per.square | cv | initials | notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TEST_P1432 | before sonication | 20210321 | 3 | 194 | 232 | 200 | 176 | 195 | 182 | 65.5 | 6.517497816 | 9.950378345 | DMB | 1 mL aliquot of adult tissue slurry from Emmas Holobiont Integration samples; sonicator test samples | ||||||
| TEST_M2184 | before sonication | 20210321 | 2 | 119 | 144 | 147 | 126 | 156 | 115 | 128 | 135 | 66.875 | 7.170126518 | 10.72168451 | DMB | 1 mL aliquot of adult tissue slurry from Emmas Holobiont Integration samples; sonicator test samples | ||||
| TEST_P1170 | before sonication | 20210321 | 6 | 109 | 122 | 148 | 136 | 138 | 133 | 21.83333333 | 2.275473089 | 10.42201415 | DMB | 1 mL aliquot of adult tissue slurry from Emmas Holobiont Integration samples; sonicator test samples | ||||||
| TEST_P1432 | 1 minute | 20210321 | 3 | 189 | 194 | 206 | 211 | 210 | 169 | 65.5 | 5.377938473 | 8.210593089 | DMB | symbionts look good, consistent counts | ||||||
| TEST_M2184 | 1 minute | 20210321 | 2 | 142 | 112 | 135 | 125 | 131 | 127 | 137 | 138 | 65.4375 | 4.761733628 | 7.276765811 | DMB | symbionts look good, consistent counts | ||||
| TEST_P1170 | 1 minute | 20210321 | 6 | 120 | 118 | 139 | 128 | 131 | 140 | 21.55555556 | 1.540803061 | 7.14805544 | DMB | symbionts look good, consistent counts | ||||||
| TEST_P1432 | 2 minutes | 20210321 | 3 | 229 | 222 | 193 | 213 | 71.41666667 | 5.202385493 | 7.284553782 | DMB | symbionts look good, consistent counts | ||||||||
| TEST_M2184 | 2 minutes | 20210321 | 2 | 131 | 115 | 123 | 154 | 65.375 | 8.410063416 | 12.86434175 | DMB | symbionts look good, consistent counts | ||||||||
| TEST_P1170 | 2 minutes | 20210321 | 6 | 120 | 144 | 147 | 146 | 23.20833333 | 2.14896616 | 9.259459216 | DMB | symbionts look good, consistent counts | ||||||||
| TEST_P1432 | 3 minutes | 20210321 | 3 | 210 | 230 | 196 | 210 | 70.5 | 4.662696724 | 6.613754219 | DMB | symbionts look good, consistent counts | ||||||||
| TEST_M2184 | 3 minutes | 20210321 | 2 | 135 | 126 | 121 | 126 | 63.5 | 2.915475947 | 4.591300705 | DMB | symbionts look good, consistent counts | ||||||||
| TEST_P1170 | 3 minutes | 20210321 | 6 | 110 | 121 | 127 | 146 | 21 | 2.512929528 | 11.96633109 | DMB | symbionts look good, consistent counts | ||||||||
| TEST_P1432 | 4 minutes | 20210321 | 3 | 202 | 222 | 212 | 180 | 68 | 5.987641593 | 8.805355285 | DMB | symbionts look good, consistent counts | ||||||||
| TEST_M2184 | 4 minutes | 20210321 | 2 | 128 | 121 | 114 | 103 | 58.25 | 5.330728531 | 9.151465289 | DMB | symbionts look good, lower counts | ||||||||
| TEST_P1170 | 4 minutes | 20210321 | 6 | 132 | 119 | 148 | 136 | 22.29166667 | 1.992462649 | 8.938150201 | DMB | symbionts look good, consistent counts | ||||||||
| TEST_P1432 | 5 minutes | 20210321 | 3 | 173 | 209 | 210 | 211 | 66.91666667 | 6.172669751 | 9.224413077 | DMB | saw a few lysed/degraded cells, lower counts | ||||||||
| TEST_M2184 | 5 minutes | 20210321 | 2 | 112 | 114 | 128 | 121 | 59.375 | 3.63719214 | 6.125797289 | DMB | saw a few lysed/degraded cells, lower counts | ||||||||
| TEST_P1170 | 5 minutes | 20210321 | 6 | 122 | 133 | 121 | 132 | 21.16666667 | 1.062840359 | 5.021293037 | DMB | saw a few lysed/degraded cells, lower counts |
Notes for 20210321
- I started to notice that cells started to lyse and degrade after a total time in sonicator of 15 minutes. The next step was to test Hollie’s 2018 Mcap developmental timeseries samples, making sure to test samples from eggs to larvae.
Symbiont count data for test samples completed on 20210323
- I used Hollie’s 2018 Mcap developmental timeseries samples from fertilized eggs, gastrula, and larvae stages.
| tube.ID | time.sonicated | date.counted | num.squares | count1 | count2 | count3 | count4 | count5 | count6 | count7 | count8 | # individuals | volume (uL) | avg.per.square | cells.uL | avg.per.individual | std.per.square | cv | initials | notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TEST_84 | 10 minutes | 20210323 | 9 | 24 | 46 | 43 | 30 | 26 | 31 | 3.703703704 | 1.007788599 | 27.21029217 | DMB | were not breaking up in 5 minutes, used plastic pestle to breakdown, sonicated for another 10 minutes and that worked | ||||||
| TEST_55 | 10 minutes | 20210323 | 8 | 103 | 111 | 109 | 112 | 106 | 110 | 13.5625 | 0.423895624 | 3.125497688 | DMB | were not breaking up in 5 minutes, used plastic pestle to breakdown, sonicated for another 10 minutes and then had to homogenize | ||||||
| TEST_35 | 10 minutes | 20210323 | 9 | 67 | 44 | 48 | 53 | 60 | 45 | 5.87037037 | 1.01206711 | 17.24025992 | DMB | were not breaking up in 5 minutes, used plastic pestle to breakdown, sonicated for another 10 minutes and that worked |
Notes for 20210323
- I noticed that the sonicator seemed to be more just mixing the samples but did not seem strong enough to breakdown the samples on its own. We decided to use a plastic pestle to break apart the initial layer of the individuals and sonicated them again. It seemed to work on two of the samples but not the last one and I did not want to reach the limit where the cells would start to lyse. I instead used the plastic pestle, the homogenizer, and the vortex to get the samples to fully homogenize within the tubes. We decided this was the best step moving forward since the sonicator did not have a setting to adjust the frequency.
Symbiont count data for test samples and Ariana samples completed on 20210325
- I used Hollie’s 2018 Mcap developmental timeseries samples from fertilized eggs, cleaving eggs, and swimming larvae stages to test the plastic pestle, homogenizer, and vortex sequence of steps again before moving forward. Once we were satisfied with how well the samples were homogenized using this method, I moved forward with counts for Ariana’s samples.
Symbiont count data with Hollie’s samples
| tube.ID | time.sonicated | date.counted | num.squares | count1 | count2 | count3 | count4 | count5 | count6 | count7 | count8 | # individuals | volume (uL) | avg.per.square | cells.uL | avg.per.individual | std.per.square | cv | initials | notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TEST_38 | none | 20210325 | 9 | 85 | 94 | 89 | 108 | 87 | 85 | 19 | 500 | 10.14814815 | 5074.074074 | 133528.27 | 0.980467681 | 9.661542837 | DMB | testing pestle, homogenizing, vortexing; fertilized eggs | ||
| TEST_56 | none | 20210325 | 8 | 124 | 121 | 124 | 125 | 112 | 110 | 55 | 500 | 14.91666667 | 7458.333333 | 67803.03 | 0.827898947 | 5.550160539 | DMB | testing pestle, homogenizing, vortexing; cleaving eggs | ||
| TEST_348 | none | 20210325 | 7 | 112 | 105 | 109 | 111 | 104 | 106 | 18 | 300 | 15.4047619 | 4621.428571 | 77023.81 | 0.473085113 | 3.071031643 | DMB | testing pestle, homogenizing, vortexing; swimming larvae | ||
| TEST_344 | none | 20210325 | 8 | 104 | 103 | 106 | 100 | 105 | 104 | 38 | 500 | 12.95833333 | 6479.166667 | 85252.19 | 0.25819889 | 1.992531625 | DMB | testing pestle, homogenizing, vortexing; swimming larvae |
Symbiont count data with Ariana’s samples (n=4)
| tube.ID | lifestage | date.counted | num.squares | count1 | count2 | count3 | count4 | count5 | count6 | count7 | count8 | avg.per.square | std.per.square | cv | total.volume.ul | num.individuals | initials | notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1-1 | Egg Fertilized | 20210325 | 2 | 135 | 133 | 157 | 170 | 174 | 170 | 175 | 173 | 80.4375 | 8.756375229 | 10.88593657 | 500 | 356 | DMB | |
| H2-1 | Egg Fertilized | 20210325 | 2 | 179 | 167 | 165 | 171 | 171 | 169 | 85.16666667 | 2.422120283 | 2.843976849 | 500 | 259 | DMB | |||
| H3-1 | Egg Fertilized | 20210325 | 2 | 160 | 154 | 163 | 171 | 155 | 170 | 81.08333333 | 3.625143675 | 4.470886342 | 500 | 256 | DMB | |||
| H4-1 | Egg Fertilized | 20210325 | 2 | 156 | 159 | 174 | 170 | 177 | 171 | 83.91666667 | 4.212085786 | 5.019367371 | 500 | 269 | DMB |
Notes for 20210325
- Happy with how the sample counts turned out and how homogenized they were for the symbiont counts. There was some tissue remains left within the samples under the microscope but not enough to disturb the accuracy of the counts.
Symbiont count data for Ariana samples completed on 20210328 (n=6)
| tube.ID | lifestage | date.counted | num.squares | count1 | count2 | count3 | count4 | count5 | count6 | count7 | count8 | avg.per.square | std.per.square | cv | total.volume.ul | num.individuals | initials | notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H5-1 | Embryo 1 | 20210328 | 2 | 160 | 184 | 193 | 187 | 189 | 190 | 91.91666667 | 6.028404985 | 6.558554834 | 500 | 254 | DMB | |||
| H6-1 | Embryo 1 | 20210328 | 2 | 192 | 188 | 181 | 186 | 187 | 182 | 93 | 2.024845673 | 2.177253412 | 500 | 232 | DMB | |||
| H7-1 | Embryo 1 | 20210328 | 2 | 186 | 180 | 179 | 186 | 183 | 179 | 91.08333333 | 1.655797894 | 1.817893388 | 500 | 306 | DMB | |||
| H8-1 | Embryo 1 | 20210328 | 2 | 187 | 179 | 169 | 170 | 156 | 178 | 86.58333333 | 5.342440142 | 6.170286978 | 500 | 214 | DMB | |||
| H9 | Larvae 1 | 20210328 | 2 | 112 | 119 | 120 | 118 | 107 | 125 | 58.41666667 | 3.184598352 | 5.451523571 | 500 | 218 | DMB | |||
| H10 | Larvae 1 | 20210328 | 2 | 137 | 130 | 129 | 122 | 131 | 136 | 65.41666667 | 2.709551008 | 4.141988803 | 500 | 294 | DMB |
Notes for 20210328
- All the counts went well and the CV’s all checked out.
Symbiont count data for Ariana samples completed on 20210330 (n=10)
| tube.ID | lifestage | date.counted | num.squares | count1 | count2 | count3 | count4 | count5 | count6 | count7 | count8 | avg.per.square | std.per.square | cv | total.volume.ul | num.individuals | initials | notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H11 | Larvae 1 | 20210330 | 3 | 112 | 116 | 110 | 110 | 108 | 105 | 36.72222222 | 1.236781965 | 3.367938786 | 500 | 189 | DMB | |||
| H12 | Larvae 1 | 20210330 | 3 | 135 | 143 | 143 | 156 | 151 | 155 | 49.05555556 | 2.735906648 | 5.577159644 | 500 | 119 | DMB | |||
| H13 | Larvae 2 | 20210330 | 3 | 129 | 131 | 128 | 125 | 131 | 130 | 43 | 0.76011695 | 1.767713837 | 500 | 105 | DMB | |||
| H14 | Larvae 2 | 20210330 | 5 | 115 | 117 | 115 | 118 | 110 | 111 | 22.86666667 | 0.640832792 | 2.802475765 | 500 | 38 | DMB | |||
| H15 | Larvae 2 | 20210330 | 5 | 122 | 115 | 116 | 125 | 120 | 130 | 24.26666667 | 1.129011367 | 4.652519369 | 500 | 49 | DMB | |||
| H16 | Larvae 2 | 20210330 | 5 | 128 | 130 | 123 | 129 | 112 | 110 | 24.4 | 1.775387282 | 7.276177384 | 500 | 40 | DMB | |||
| H17 | Larvae 3 | 20210330 | 3 | 100 | 109 | 103 | 106 | 101 | 105 | 34.66666667 | 1.115546702 | 3.217923179 | 500 | 66 | DMB | |||
| H18 | Larvae 3 | 20210330 | 3 | 120 | 113 | 107 | 111 | 112 | 107 | 37.22222222 | 1.600925658 | 4.300994306 | 500 | 88 | DMB | |||
| H19 | Larvae 3 | 20210330 | 3 | 115 | 114 | 106 | 101 | 103 | 102 | 35.61111111 | 2.059305888 | 5.782762244 | 500 | 76 | DMB | |||
| H20 | Larvae 3 | 20210330 | 4 | 105 | 110 | 110 | 120 | 121 | 106 | 28 | 1.724818831 | 6.160067254 | 500 | 38 | DMB |
Notes for 20210330
- All the counts went well and the CV’s all checked out.
Symbiont count data for Ariana samples completed on 20210401 (n=20)
| tube.ID | lifestage | date.counted | num.squares | count1 | count2 | count3 | count4 | count5 | count6 | count7 | count8 | avg.per.square | std.per.square | cv | total.volume.ul | num.individuals | initials | notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H21 | Larvae 4 | 20210401 | 1 | 101 | 100 | 102 | 101 | 105 | 102 | 101.8333333 | 1.722401424 | 1.691392561 | 500 | 208 | DMB | |||
| H22 | Larvae 4 | 20210401 | 2 | 120 | 146 | 143 | 135 | 146 | 151 | 70.08333333 | 5.598362856 | 7.988151519 | 500 | 118 | DMB | |||
| H23 | Larvae 4 | 20210401 | 2 | 147 | 148 | 140 | 143 | 134 | 154 | 72.16666667 | 3.473710792 | 4.813456063 | 500 | 107 | DMB | |||
| H24 | Larvae 4 | 20210401 | 2 | 180 | 179 | 181 | 186 | 193 | 180 | 91.58333333 | 2.709551008 | 2.95856343 | 500 | 176 | DMB | |||
| H25 | Larvae 5 | 20210401 | 3 | 107 | 117 | 114 | 109 | 108 | 105 | 36.66666667 | 1.5202339 | 4.146092455 | 500 | 55 | DMB | |||
| H26 | Larvae 5 | 20210401 | 3 | 102 | 104 | 116 | 110 | 100 | 108 | 35.55555556 | 1.962613526 | 5.519850541 | 500 | 48 | DMB | |||
| H27 | Larvae 5 | 20210401 | 4 | 138 | 113 | 110 | 112 | 112 | 124 | 29.54166667 | 2.731376332 | 9.245843719 | 500 | 40 | DMB | |||
| H28 | Larvae 5 | 20210401 | 4 | 121 | 117 | 111 | 122 | 118 | 125 | 29.75 | 1.21449578 | 4.082338757 | 500 | 47 | DMB | |||
| H29 | Larvae 6 | 20210401 | 6 | 115 | 111 | 110 | 112 | 116 | 118 | 18.94444444 | 0.523520844 | 2.763453135 | 500 | 16 | DMB | |||
| H30 | Larvae 6 | 20210401 | 7 | 114 | 112 | 108 | 113 | 121 | 115 | 16.26190476 | 0.608891041 | 3.744278727 | 500 | 19 | DMB | |||
| H31 | Larvae 6 | 20210401 | 6 | 118 | 121 | 115 | 123 | 120 | 116 | 19.80555556 | 0.510083508 | 2.575456703 | 500 | 21 | DMB | |||
| H32 | Larvae 6 | 20210401 | 6 | 107 | 105 | 111 | 109 | 110 | 109 | 18.08333333 | 0.361324723 | 1.998109068 | 500 | 16 | DMB | |||
| H33 | Recruit 1 | 20210401 | 7 | 101 | 100 | 102 | 104 | 103 | 100 | 14.52380952 | 0.233284737 | 1.606222782 | 500 | 13 | DMB | |||
| H34 | Recruit 1 | 20210401 | 6 | 117 | 121 | 118 | 119 | 121 | 118 | 19.83333333 | 0.278886676 | 1.406151305 | 500 | 18 | DMB | |||
| H35 | Recruit 1 | 20210401 | 6 | 114 | 113 | 116 | 110 | 112 | 112 | 18.80555556 | 0.340206909 | 1.809076619 | 500 | 20 | DMB | |||
| H36 | Recruit 1 | 20210401 | 5 | 118 | 120 | 116 | 117 | 110 | 116 | 23.23333333 | 0.67428975 | 2.902251434 | 500 | 33 | DMB | |||
| H37 | Recruit 2 | 20210401 | 6 | 110 | 115 | 108 | 110 | 108 | 107 | 18.27777778 | 0.479196859 | 2.621745733 | 500 | 16 | DMB | |||
| H38 | Recruit 2 | 20210401 | 9 | 110 | 120 | 111 | 113 | 108 | 115 | 12.53703704 | 0.473581921 | 3.777462881 | 500 | 12 | DMB | |||
| H39 | Recruit 2 | 20210401 | 9 | 89 | 88 | 92 | 75 | 93 | 96 | 9.87037037 | 0.817755636 | 8.284953908 | 500 | 6 | DMB | |||
| H40 | Recruit 2 | 20210401 | s | 84 | 85 | 71 | 77 | 86 | 91 | 9.148148148 | 0.794010501 | 8.679467009 | 500 | 7 | DMB |
Notes for 20210401
- All the counts went well and the CV’s all checked out. All symbiont counts completed.
Coral Plugs Protocol
Process all of the physiological data (i.e. size and symbiont counts) for the Montipora capitata (vertical spawner) coral plugs with recruits (n=8).
Notes for 20211018
First - I took photos of each individual plug for surface area measurements.
Materials
- Glass pipette
- Dissecting light microscope
- OMAX digital microscope camera
- ImageJ software
- OMAX 0.01 mm stage micrometer
- ToupView software (windows operating system only)
- Cell culture dish
Protocol Steps
- I aligned the coral plug underneath the dissecting microscope with the light on medium to high and positioned the coral plug next to the 0.01 mm stage micrometer so that the total length of the scale could be seen within the photo along with all the individuals.
- it is very important to have the scale micrometer within each individual photo as the zoom and focus may change on the microscope in between samples and you will need it to calibrate your photos later on in ImageJ
- Once the stage micrometer and coral plugs were focused and clear in view, I used the ToupView software to snap a photo and save it to the windows computer.
- I uploaded the photos to Google Drive and then when I was ready to size the individuals I would open them in ImageJ.
- Refer to the ImageJ manual with any questions on how to calibrate or how to use the freehand tool
- I selected the Straight Line Selection Tool in ImageJ and zoomed in to the scale bar on the stage micrometer. I then carefully drew a line from each end of the 0.01 mm scale bar. I then set the number of pixels to a known distance of 1.0 and the unit length to mm. I also click on global so that it will be set for as long as I have this image open.

- I then use the freehand selections tool to carefully draw a circle around the entire outline of each recruit on the top of the coral plugs.
- make sure to go slow and zoom in as far as possible with the individual still in focus when doing this step.
- After outlining the individual, I press command M (measure) and a small data box will pop up with the calculated surface area of the individual in mm.
- make sure to keep an eye on these measurements and if you see them fluctuating or having large numbers, do the set scale step above again
- I then save the photo again and transfer the measured values into a csv document.
- All labeled images were uploaded here.
Second - I counted the number of endosymbionts within each coral recruit per plug for symbiont density.
Materials
- Glass pipette
- OMAX compound light microscope
- Vortex
- Haemocytometer
- Hand held tally counter
- 200 uL pipette
- 1.5 mL microcentrifuge tubes
- Homogenizer
- 70% ethanol
- 10% bleach
- DI water
Protocol Steps
Followed the E5 Symbiodiniaceae Cell Density Counting Protocol with a few modifications for removing recruits from coral plugs.
- First, I put 100 uL of Type II DI water into 8 individually labeled microcentrifuge tubes.
- I then sterilized a scalpel with 10% bleach, DI water, and 70% ethanol before carefully removing each individual coral recruit from the coral plugs. I lightly applied pressure underneath each coral recruit with the scalpel and slowly moved the scalpel underneath the coral recruit until it was fully removed and could be placed into the labeled 1.5 mL microcentrifuge tube.
- I used the small attachment head on the homogenizer and individually homogenized each microcentrifuge tube sample for 30 seconds and sterilized the homogenizer in between each sample with the 10% bleach, DI water, and 70% ethanol solutions.
- I then followed the E5 Symbiodiniaceae Cell Density Counting Protocol and recorded the final endosymbiont counts here.
Symbiont count data for coral plug samples completed on 20211018
| lifestage | date.counted | num.squares | count1 | count2 | count3 | count4 | count5 | count6 | count7 | count8 | avg.per.square | std.per.square | cv | total.volume.ul | num.individuals | initials |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plug | Mcap1 | 20211018 | 2 | 142 | 132 | 123 | 137 | 113 | 150 | 66.41666667 | 6.658953872 | 10.02602841 | 100 | NA | DMBP | |
| Plug | Mcap2 | 20211018 | 2 | 136 | 140 | 141 | 133 | 129 | 145 | 68.66666667 | 2.909753712 | 4.237505406 | 100 | NA | DMBP | |
| Plug | Mcap4 | 20211018 | 2 | 150 | 141 | 147 | 156 | 151 | 143 | 74 | 2.75680975 | 3.725418582 | 100 | NA | DMBP | |
| Plug | Mcap5 | 20211018 | 2 | 189 | 176 | 200 | 196 | 195 | 201 | 96.41666667 | 4.641300105 | 4.813794405 | 100 | NA | DMBP | |
| Plug | Mcap6 | 20211018 | 2 | 200 | 196 | 189 | 176 | 191 | 201 | 96.08333333 | 4.619704175 | 4.808018222 | 100 | NA | DMBP | |
| Plug | Mcap8 | 20211018 | 2 | 189 | 199 | 187 | 175 | 202 | 187 | 94.91666667 | 4.841659495 | 5.100958204 | 100 | NA | DMBP | |
| Plug | Mcap9 | 20211018 | 2 | 172 | 180 | 198 | 200 | 186 | 191 | 93.91666667 | 5.370443805 | 5.718307512 | 100 | NA | DMBP | |
| Plug | Mcap10 | 20211018 | 2 | 198 | 204 | 191 | 196 | 199 | 186 | 97.83333333 | 3.172801076 | 3.243067539 | 100 | NA | DMBP |

